
 |
Department of Water Affairs and Forestry |
| Institute for Water Quality Studies |
| Home | Contact us | Last updated : 10 July 2001 |
The salinity in the Roodeplaat Dam is only expressed as electrical conductivity (EC). The Roodeplaat Dam does not seem to have problems regarding the salt content in the impoundment. The EC readings varied between 30 mS/m and 60mS/m for the whole study period (Figure 8). The water in the Roodeplaat Dam is, therefore, within the South African Water Quality Guideline for aquatic ecosystems of 450 mg/l total dissolved salts (TDS) (an EC of ±70 mS/m) (DWAF 1996).
The composition of the inorganic salts governs the effects of the TDS. The proportional concentrations of the major ions affect the buffering capacity of the water and hence the metabolism of organisms in the water bodies. Most commonly, the relative concentrations of the major ions tend to be the same as was found in the Roodeplaat Dam (Na+ (38.4 mg/l ) > Ca2+ (24.7 mg/l ) > Mg2+ (15.2 mg/l ) > K+ (7.19 mg/l ). The anions show the tendency where Cl- (40.5 mg/l ) > HCO3- > SO42- (36.7 mg/l ) > CO32-). The water in the Roodeplaat Dam can, therefore, be described as hardwater where Cl- predominates. This phenomenon is typically when large volumes of sewage effluent contribute to the water resource. This anion is usually not dominant in open lake systems, but pollution sources of chlorides can modify the natural concentrations (WETZEL, 1983) which is most probably the case in the Roodeplaat Dam. High mean Na+ (40 mg/l ) concentrations were found to be optimal for several cyanobacteria (WETZEL, 1983). The high Na+ concentrations in the Roodeplaat Dam are, therefore, contributing to the development of the cyanobacterial blooms in the Roodeplaat Dam.
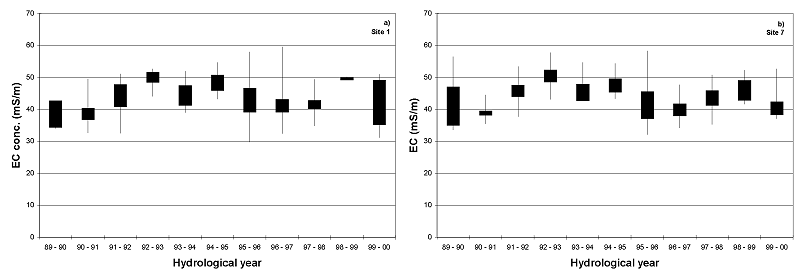
Figure 8. The EC readings in the Roodeplaat Dam at a) Site 1 and Site 7 for the period 1989 to 2000.